Home › Electrical Engineering Forum › General Discussion › Introduction to Semi Conductors
- This topic has 0 replies, 1 voice, and was last updated 10 years, 1 month ago by
admin.
-
AuthorPosts
-
2014/10/03 at 4:18 pm #11198
admin
KeymasterNew series of articles by our devoted member Nasir. This time he will talk about Semi Conductors in almost 10 articles.
Remember you too can send us articles about whatever you want by sending us a mail!
Introduction
Here we are going to start a detailed tutorial on power semiconductor switching devices, which will give you a brief but explanatory overview of semiconductors and the semiconductor switching devices used in power electronic circuits. So taking a traditional start with the definition of Power semiconductor switching devices, the first question that comes to our mind is that ‘what basically power semiconductor switching devices are’?
The term is self-explanatory and speaks for itself, that power semiconductor switching devices are the semiconductor switching devices which can have only two states, i.e. on or off, hence acting like a switch.
- They are used in power electronic circuits and like a switch they cannot have a third state.
- They are becoming extremely popular now a days due to their fast switching frequency and appealing properties
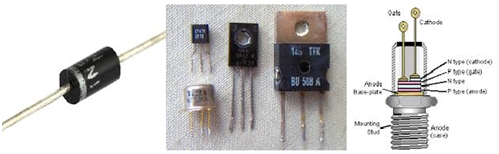
- Some commonly used examples of power semiconductor switching devices include diodes, transistors, thyristors etc. But first I would like to revise what basically semiconductors are.
Semiconductors
As the name suggests, semiconductor is a material which cannot conduct completely, instead its conduction ability lies between that of an insulator and a conductor. This means that they have a resistivity too low to be called an insulator but at the same time, too high to be called a conductor.
When talking about conduction we know that electrons are the primary charge carries which tend to conduct in conductors.
- Elements which are classified as conductors have free electrons or charge carriers in their outermost shell which are free to move and conduct.
- On the other hand, insulators have a fully filled valence shell so have no free electrons or charge carriers to conduct hence making them unreactive.
So now the question arises that, how do semiconductors fit in this scenario if it has to be one way or the other. The answer is that there is a third way as well, which is adopted by the semiconductors.
Doping
Semiconductors belong to the 4th group of the periodic table, which means that they have four electrons in their outer most or valence shells. The resistivity offered by the semiconductor in this state can be changed by adding an impurity which changes the number of charge carries in the atom. This process of adding an impurity is called doping but is such that only one atom of the impurity is added to a million semiconductor atoms.
Atoms commonly used as semiconductors include Silicon, Germanium etc. These in their pure form are known as Intrinsic Semiconductors. After the addition of impurities their resistance and electrical properties change and they are known as extrinsic semiconductors.
Types of Doped Semiconductors
There are two different ways of adding an impurity to the semiconductor atom. The types of doped semiconductors formed after the addition of the impurity are:
- N-type material
- P-type material
N-type Materials:
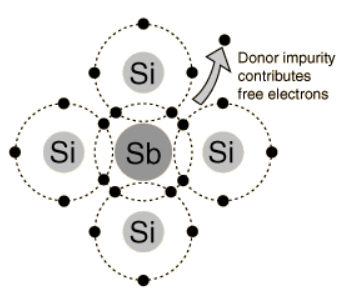
If a silicon or germanium atom in its pure form is doped with an element of group five in a small amount, such as antimony, arsenic or phosphorus, these elements having 5 electrons in their outermost shell react such that they form a covalent bond with the four electrons of silicon, and one electron is left free as a mobile charge carrier, which improves the conduction ability to some extent.
The resultant material is known as an n-type semiconductor.
P-type Materials:
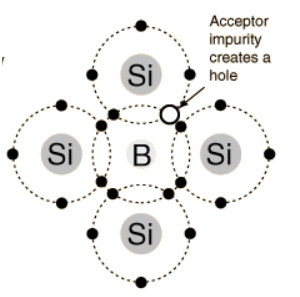
If a silicon or germanium atom in its pure form is doped with an element of group three in a small amount, such as indium, gallium or boron, these elements having 3 electrons in their outermost shell react such that they form a covalent bond with the three electrons of silicon, and one hole is left free as a mobile charge carrier, which improves the conduction ability to some extent.
The resultant material is known as a p-type semiconductor.
Conclusion
This was the basic theory of semiconductors and the conduction concepts behind them. Since now you are familiar with them so in the next article I would discuss about diodes which are one of the basic power semiconductor switching devices.
Nasir.
What do you think of this 1st article from the Semi Conductor tutorial series?
-
AuthorPosts
- You must be logged in to reply to this topic.