Home › Electrical Engineering Forum › General Discussion › Graphene can replace transparent electrical conductors in displays!
- This topic has 0 replies, 1 voice, and was last updated 10 years, 1 month ago by
Steven Mill.
-
AuthorPosts
-
2015/01/08 at 5:32 pm #11225
Steven Mill
ParticipantHi, this is Steven Mill. As you know, I love reporting electrical improvements and other news linked to the subject. Did you know graphene can replace transparent electrical conductors?
Why use transparent electrical conductors in displays in the first place?
Most of the flat screen TV’s, laptops, desktops, mobile phones and other electrical or electronic devices that need bright screens, rely on transparent electrical conductors for effective display. The reason for using transparent conductors for this purpose is, they do not obstruct the back-end illumination that lights up the front of the screen, resulting in a brighter and sharper display.
Indium Tin Oxide (ITO) is the material used in manufacturing such transparent conductors, which is not only very expensive but also very fragile. To overcome this problem, many researchers have been trying to manufacture transparent conductors with various metal alloys that can take the place of TIO, until the recent break through was discovered.What is Graphene?
A thin sheet consisting of a single layer of Carbon atoms is known as Graphene. Though scientists knew of its theoretical existence from the 18th Century, Graphene was available for practical experiments only in the last decade.
In 2004, two scientists named Andre Geim and Konstantin Novoselov were able to create Graphene from a Graphite lump, by using an adhesive tape. They were awarded Nobel Prize in the year 2010 for being able to isolate graphene. Such was the importance of the discovery of this Carbon allotrope.
Why Graphene? How effectively can it replace ITO in manufacturing transparent electrical conductors?
According to Guangxin Ni, a PhD researcher in the team, “When mixed with ferroelectric polymer, Graphene has high levels of electrical conductivity, and is also very flexible when compared to ITO. It can also be used to create thin foils of conductors. Graphene’s malleable and flexible nature allows it to serve as an effective conductor even in foldable displays, as well as solar cells.”
Toughness
It should be noted that, irrespective of its thin structure, Graphene is very tough in nature. This is because; bonds between the Carbon atoms in Graphene, despite being in a single layer, are very strong and never break under normal circumstances.
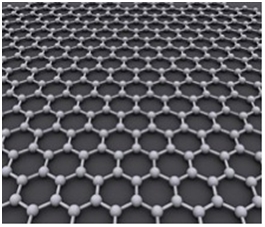
[caption id="attachment_8858" align="aligncenter" width="265"]
Graphene – Carbon Atoms Bonding Structure[/caption]
Electrical Conductivity
In pure state, Graphene is not very conductive. This is because, all the electrons in the allotrope are strongly bonded and there are only a few electrons available to carry electricity. One can overcome this problem by inducing voltage, but then again, this is an unwanted method, as it consumes electricity.
Then how is it effective? Graphene could be a bad electrical conductor when in pure state. But, when mixed with an active ferroelectric polymer, such as copper, it can outplay the best electrical conductors.
Graphene and Ferroelectric Polymer
The research team discovered that, when layer of Graphene is formed on a thin ferroelectric polymer sheet that has high electrical conductance, and then is covered on the top by the same polymer solution, Graphene’s conductivity increases by twelve folds. This is because; the electric field of the ferroelectric polymer induces electrical charges in Graphene and turns it into a highly conductive material!
Is it safe?
One should understand that, this process does not cause any harm to the flexibility or rigidness of Graphene. It also does not compromise with the high optical transparency of the material. In fact, using polymer helps in inducing electric charges into Graphene, almost indefinitely, thus making it a permanent transparent electrical conductor.
In conclusion, the research team claims that, “Graphene has not only the capability of replacing the expensive and delicate transparent electrical conductors, but can also replace the conventional batteries used in cell phones, laptops and even cars, because of its low weight, considerably less charging time and prolonged charge retaining capacity.”
Steven Mill.
What’s your opinion on that finding?
-
AuthorPosts
- You must be logged in to reply to this topic.

 Graphene – Carbon Atoms Bonding Structure[/caption]
Graphene – Carbon Atoms Bonding Structure[/caption]