
In todays modern aircrafts nickel cadmium and lithium ion cells are often in use. The old type of lead-acid batteries in commercial use.
Matching diagrams of capacity and other characteristics. Which batteries are the best? And why?
Lead-acid batteries
The lead-acid cells are consisted of two electrode, positive and negative. Electrodes are made of lead-antimony alloy grid plates.
We have many plates in electrode, packed with space, which is filled with active lead material. The plates are interconnected so we have facing the positive and negative plate in the cell. Plates don’t have contact and for that purpose we have separators in cell case.
Separators are made from good insulating material, which has ability to provide unhampered of electrolyte on plates surface. So, we have one positive and negative electrode with their palates. One lead-acid cell is presented on the Picture 1.
Chemical action
Positive electrode is made of lead-antimony alloy grid into which lead peroxide paste (PbO2) is imprinted under high pressure.
Negative electrode is made of pure Pb forced into grid. During the discharge, chemical reaction occurred, in which the electrons are transferred from negative electrode to positive and lead sulphate (PbSO4) is formed on both plates. The electrolyte is mix of sulphuric acid (H2SO4) and water.
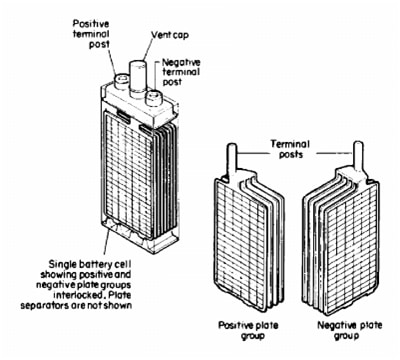
Picture 1. Lead-Acid cell
Nickel-Cadmium batteries
Like lead-acid cells, here we have two plates. Positive is composed of nickel hydroxide Ni(OH)2, and negative from cadmium hydroxide Cd(OH)2 with electrolyte potassium hydroxide (KOH).
Plates are produced in sintering process. Sintering is a process where materials are infused into plates during chemical reaction.
With these process and construction, the maximum quantity of active material in plates has been achieved. After sintering plates are formed in right dimension, similar shapes as lead-acid cells.
Chemical action
When charges, negative plate is losing oxygen and it is now metal cadmium. On the other side, positive electrode accept all oxygen. So, oxygen is disappeared from negative electrode, and positive electrode pick up the oxygen.
One typical nickel cadmium cell is presented on the Picture 2. On the Picture 2 you can see one typical nickel cadmium cell. The cell emits gas in process of charging. This gas is cased by decomposition of water consisted in the electrolyte into hydrogen at the negative and oxygen at the positive electrode.
So, in maintenance it is necessary to check periodically level of electrolyte.
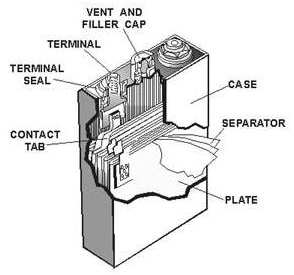
Picture 2. Nickel-Cadmium cell
Lithium ion batteries
Lithium is powerful. The lightest of all metals, lithium has very good utilization and provide excellent electromagnetic characteristics. Nowadays, we have this batteries everywhere, in smartphones, laptops and many others devices.
How does lithium work?
We have positive electrode made from lithium metal oxide (LiO2) and negative electrode from porous carbon(graphite). As you can see from the Picture 3., during the process of charging ions are transferred from negative to positive electrode through electrolyte and separator. Discharging is going vice versa.
In process of charging, positive lithium ions pass from cathode, through separator into the graphite , where they are stored. So in cathode, during the oxidation we have lithium-ions and electrons. When ions and electrons are transferred to the canode they react with graphite.
At the end Li Oxide and graphite made lithium-graphite plus Lithium Oxide. When discharge, energy is removed from the cell and lithium ions move from anode via electrolyte, through separator, back to cathode. Figure 3 illustrates the process.
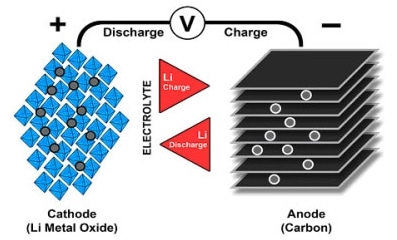
Picture 3. Lithium-ion batteries. Process of charging and discharging
Matching characteristic of batteries
If we talk about batteries in general, we think about capacity. So, capacity is total amount of available energy. It depends directly depends on the material and its amount available for chemical reaction.
Typically, capacity is measured in ampere-hours, and one ampere hour is defined as maximum current, which is delivered for known time period.
Beside capacity, there is discharge rate. The time taken to discharge is discharge rate and capacity of batteries are matched with this process. So, in case of discharging 5 A during 9 hours, capacity is 45Ah. Please take a look on Pictures 4 and 5. You will see the difference between three battery types.
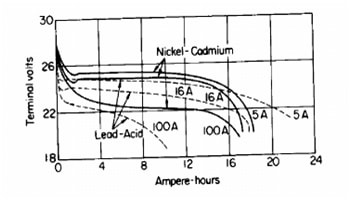
Picture 4. Discharge rate of NiCd and Pb batteries
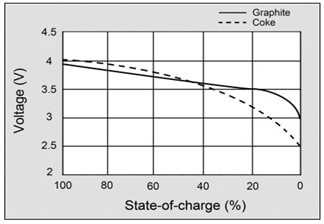
Picture 5. State of charge Li-ion batteries
A battery should have a flat voltage curve in the usable discharge range.
Todays lithium-ion cells have high energy per cell, much more higher than nickel-based cells. As you can see from Picture 5., The load characteristics are excellent and it is provided good utilization of voltage. So, in range of 4-3V discharging characteristic is flat. Compare it with characteristic of nickel-based and lead-acid cells. You we see that characteristic are not so good.
Nickel-cadmium batteries have memory effect. This effect happens during maintenance, and memory effect occurs when cell is not fully discharged. In that scenario, cell keeps and remember value when it starts to charge, and that value is new – 0V.
Otherwise lithium-ion cells doesn’t have memory effect, and there is no need for full discharge to keep cells in good conditions.
Another characteristic matched in comparison is crystalline formation in nickel-cadmium cells. It is very important to follow the manuals and to periodically check nickel-based cells in 3 months.
During this check, called ‘exercise’ full discharge of cells occurred. If we don’t do periodic checks, active material will applied in negative electrode, and forms a crystalline formation. This will reduce the surface area of active material.
However, lithium-ion cells have some disadvantages. To ensure the required safety, this batteries have to be controlled and regulated by electronics. Battery Management System is electronic system which controls lithium-ion batteries on aircrafts.
This system provides: measuring a battery’s state of charge, measuring battery state of health, provide battery protection monitoring, ensures control of discharging and protect over-discharge. The control of charging and discharging is essential, because malfunction in these operations cause overheating.
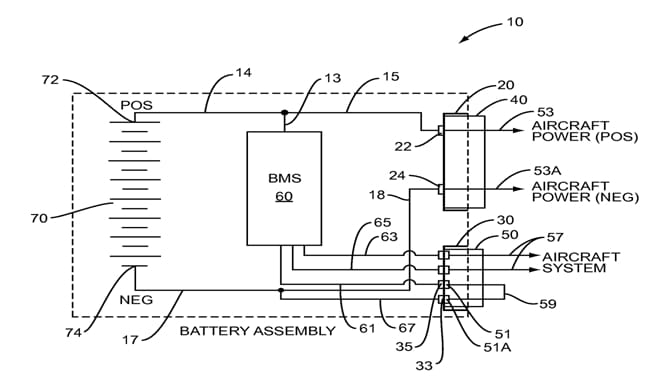
Picture 6. Battery management system
Which batteries are the best? And why?
At the end, the answer to this question can provide a final conclusion. In most todays aircrafts in use are nickel-based cells. Past years lithium-ion change the nickel-based cells. So, from Cesna to Boeing there is a trend for changing to lithium-ion. They are small in weight and dimensions, good in capacity, and confident in use with implementation of battery management system (BMS).
So, in the future it will be lithium-ion, and please see the diagram in Picture 7. that represents specific power and energy levels of lithium-ion, nickel-cadmium and lead-acid cells.
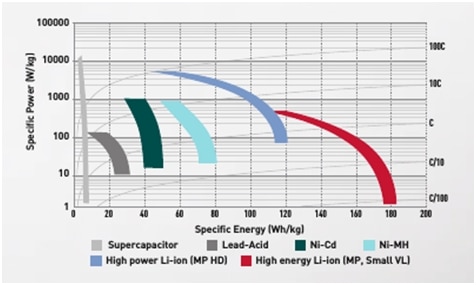
Picture 7. Comparison of cells. Specific power and Specific energy
Milan.
Do you agree with Milan’s comparison of batteries? To your opinion, which are the best?
This is post is really great! I enjoyed reading it
In addition I’m going to add a comment about the battery maintenance, beacuse the correct maintenance of batteries is essential to warn his exhaustion and to know when to replace it. With proper maintenance, we can avoid the battery failure. Each type of battery has several types of failure, depending on the type of battery. Some failures occur due to the use and others occur naturally, but proper maintained can reduce the risks of premature battery failure.
Whether it is a lead-acid battery (flooded or VRLA) or a nickel-cadmium battery, you can work following several maintenance philosophies. The batteries can be replaced when they fail or replace them after a certain time, this being a very risky option, because the maintenance is very reduced or zero and the risk of failure sooner than expected is high.
For this it is important this thread:
Industrial battery charger
Digital battery charger
Automatic battery charger
battery rectifier
Battery charger rectifier
High efficiency battery charger
Battery Monitoring System
Rectifier industrial battery charger
Battery Monitor
Amperis industrial battery control
compact industrial battery charger
battery chargers guide
Traction battery charger
Multifrequency battery charger
Hybrid battery charger
Multivoltage battery charger
High Frequency Battery Charger
Battery discharger
Battery Resistance Tester
battery discharge test equipment
Load Bank battery test
Battery analyzer
Industrial battery analyzer
Battery simulator
Industrial battery Charger Tester
Rectifier Battery Charger
Regards!